At least two atoms join to shape a particle and the particle together structures everything present around us, so we can say that the premise of all things and substances is the actual molecule.
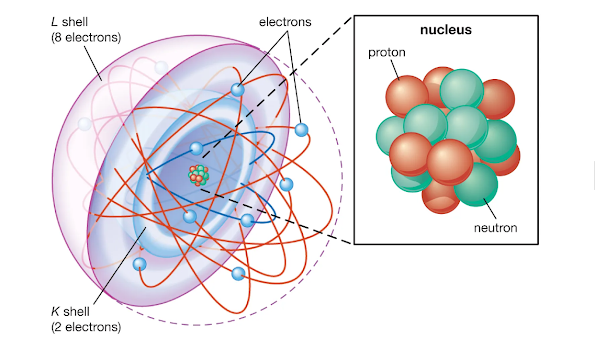
Particles comprise a tiny, decidedly charged core encompassed by a haze of adversely charged electrons. Albeit regularly the core is short of what one ten-thousandth the size of the particle, the core contains more than 99.9% of the mass of the molecule.
It is made out of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons encompassing them are extensive particles present in all normal, normally happening atoms.
The molecule is comprised of three particles
1. Electron
2. Proton
3. Neutron
Of these, protons and neutrons are found in the core of the atom, the core is the focal piece of the molecule. Where the proton has a positive charge, the electron has a negative charge and the neutron is chargeless, or at least, no charge exists on the neutron.
Molecules typically have a similar number of protons and electrons. What to note here is that protons and neutrons are additionally comprised of particles called quarks and gluons.
Structure of Atom
Atom is a Greek word that signifies "what can't be broken", on the grounds that when a molecule was found then it was considered the littlest molecule and it was accepted that the particle couldn't be broken for example from this everything It has been developed, being the littlest unit is thought of.
However, some other times when electrons, protons, and neutrons were found, it was observed that molecules are additionally comprised of different particles, and protons and neutrons are likewise comprised of quarks and gluons.
Discovery of Atom
The particle was found by John Dalton in 1803, and to make sense of the atom, he gave his atomic hypothesis, the marks of which are as per the following -
1. Each substance is comprised of tiny particles, these particles are called atoms.
2. All molecules of a similar substance are comparable in size, weight, and different properties, yet the properties of particles of various substances can be unique.
3. Molecules can't be broken, made, or obliterated yet today in this advanced time it is conceivable.
History of Atom
Democritus Introduces the Atom
The historical backdrop of the atom starts around 450 B.C. with a Greek rationalist named Democritus. Democritus considered what might occur in the event that you cut a piece of tissue, like an apple, into increasingly small pieces. He felt that a point would be reached where matter couldn't be cut into still more modest pieces. He called these "uncuttable" pieces atoms. This is where the cutting-edge term particle comes from.
Democritus was a significant rationalist. Notwithstanding, he was less compelling than the Greek logician Aristotle, who lived around 100 years after Democritus. Aristotle dismissed Democritus' concept of molecules. Aristotle thought the possibility of particles was ludicrous, as a matter of fact. Sadly, Aristotle's thoughts were acknowledged for over 2000 years. During that time, Democritus' thoughts were pretty much neglected.
John Dalton Introduces the Atom
Around 1800, a British scientific expert named John Dalton restored Democritus' initial thoughts regarding the particle. Dalton is imagined in the Figure underneath. He earned enough to pay the bills by educating and just exploring in his extra time. In any case, from his exploration results, he created perhaps the main hypothesis in science.
Quantum model of particle
Along these lines, this model was responding to many inquiries, however, it was disregarding the double idea of the issue. So a quantum model was presented in which these orbitals have more modest spaces, "orbitals". Four quantum numbers (numbers) were likewise reported to recognize them.
• The Principal Quantum Number - "n"
• Azimuthal Quantum Number - "l"
• Attractive Orbital Quantum Number - "m(l)"
• Electron Spin Quantum Number - "m(s)"
• In the event that you have any inquiry or idea connected with this subject, you can compose it in the remark beneath.
Niels Bohr nuclear model
Niels Bohr attempted to compensate for the model deficit and made sense of:
Just certain proper circles, which are called discrete circles, can move electrons. These circles have their own energy and the electrons don't lose their energy while moving around in them.
Downsides of rutherford model of the molecule
Since the electrons are turning around, their pivot is sped up. Maxwell's electromagnetic regulation expresses that any charged molecule that is sped up will in general lose its energy.
Along these lines, the electron would fall into the core after some time, which would have made the particle shaky. However, we realize that the particle is steady.
Rutherford model of Atom
Based on this investigation, a researcher named "Rutherford" did another examination, so he could know the area of the electron. He took gold foil since he needed an extremely slight layer.
Then, at that point, they quickly came down alpha particles (helium iotas without electrons) on it. The alpha molecule weighed 4u, so it had a ton of energy. He anticipated that concurring should the Thomson model, practically all alpha particles would crash into protons and return. However, the outcomes were stunning.
• Practically every one of the alpha particles went through the gold foil.
• A couple of particles bowed somewhat.
• In the assessed 12000, just a solitary molecule returned 1800.
• Accepting these realities as the premise, Rutherford gave one more model of the particle
• There is a ton of void space in the particle.
• Everything its positive charge and mass are detained in a core between the molecule.
• Every one of the electrons rotates around the core in its own circles.
• The core is a few times less than a molecule.
• We can imagine it like the planetary group, where the core is the spot of the sun and the electron is the spot of the planets.
Thomson model of the molecule
Presently the issue was to figure out the design of the molecule with these particles. Sir Thomson was the primary individual to endeavor to make sense of it. He said that the iota resembles a round Christmas cake, in which the cake is made of positive charge (protons) and the nuts in it have negative charge (electrons). We can likewise imagine it like a watermelon in which the red part is positive and the seeds are adversely charged.
How much sure and negative charge is something very similar, making the particle impartial. The mass of the molecule is ordinarily conveyed all through the circle.






0 Comments